A distillation rotary evaporator, commonly known as a rotavap, is an essential laboratory instrument used for the efficient and gentle removal of solvents from samples through distillation under reduced pressure. This sophisticated device has revolutionized how chemists concentrate solutions, isolate reaction products, and recover valuable solvents. Unlike traditional distillation setups, the distillation rotary evaporator combines multiple physical principles to achieve rapid and safe solvent removal.
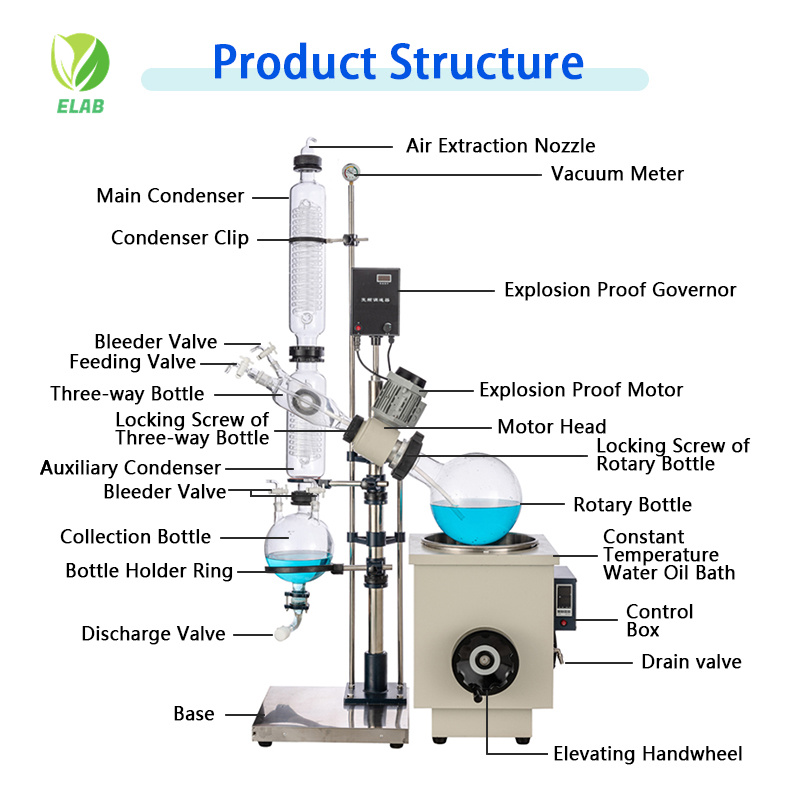
The distillation rotary evaporator operates on a simple yet elegant principle: by creating a large surface area of the liquid while simultaneously lowering its boiling point. The system consists of several key components working in harmony. The main parts include a motor unit that rotates the evaporation flask, a heated water or oil bath, a vacuum system, a condenser, and a receiving flask for collecting the distilled solvent. The rotation mechanism is particularly crucial as it continuously exposes fresh liquid surface to the vacuum and heat.
The working process of a distillation rotary evaporator begins with placing the sample solution in a round-bottom flask, which is then attached to the apparatus. The flask is partially immersed in a heated bath while being rotated continuously at speeds between 100-200 rpm. This rotation spreads the liquid as a thin film across the inner surface of the flask, dramatically increasing the surface area available for evaporation. The thin film formation is the key innovation that distinguishes this method from conventional distillation.
Simultaneously, a vacuum pump reduces the pressure inside the system, which significantly lowers the boiling point of the solvent. For example, ethanol boiling at 78°C under normal atmospheric pressure can be evaporated at room temperature under vacuum conditions. The combination of thin-film formation, reduced pressure, and controlled heating allows solvents to evaporate rapidly yet gently, preventing thermal decomposition of sensitive compounds. This three-pronged approach makes the distillation rotary evaporator far more efficient than simple heating or vacuum alone.
The solvent vapor travels upward through a vapor duct into the condenser, where it is cooled and converted back into liquid form. This purified solvent then collects in the receiving flask, while the concentrated solute remains in the original evaporation flask. Modern distillation rotary evaporator systems often include digital controls for precise regulation of temperature, rotation speed, and vacuum pressure, ensuring reproducible results and enhanced safety features. The entire process can be monitored through sight glasses and controlled automatically.
The efficiency of a distillation rotary evaporator makes it indispensable in organic chemistry, pharmaceuticals, and food science. Compared to traditional distillation methods, it offers faster processing times, lower operating temperatures, and better protection for heat-sensitive materials. By understanding these principles, researchers can optimize their solvent removal processes while maintaining sample integrity and achieving higher yields.
Wonderful! Share this Blog:



